Tungsten Oxide Thin Film Electrochromism
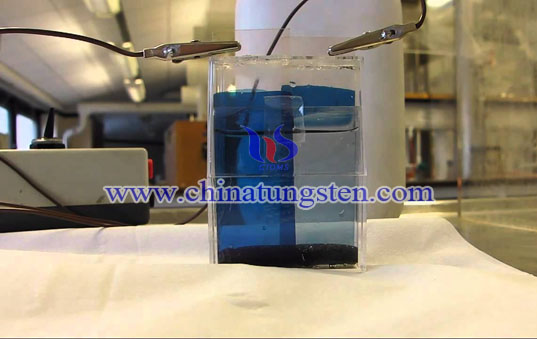
Electrochromism is the material undergoes a recersible color change under external electric field, it has been found that many transition metal oxide has electrochromic property, they can be divided into cathode material of reduction process(such as oxides of W, Mo, V, Nb, and Ti) and cathode material of oxidation process(such as oxides of Ir, Rh, Ni and Co). WO3 is one of the mostly studied cathodic coloring electrochromic material.
The most applied WO3 thin film electrochromic mechanism is Faughman’s valence transition theory. Under the effect of current, electron and anion are input into WO3 from both sides of thin film. Electron is formed into localized state by W atom, metal ion M+ stays in the area and forms into dark blue ammonium tungsten bronze MxWO3. There are W ions of different valence state existed in MxWO3, electron transite under different W atom of different valence state causes change of WO3 color. The chemical equation is:
WO3(colorless) +xM++xe→MxWO3(dark blue)
(M+ can be H+、Li+、Na+ 、Ag, 0<x<1)
Electrochromic property of WO3 has been made into different electrochromic device and is applied in real life. There are many advantages of electrochromic device, transmittance can change continuously in a wide range and can be adjusted manually. Drawing voltage is low(1-2V), low power consumption. There is no restrictions on the viewing angle on the display. During storage it does not consuming electricity. These features make WO3 electrochromic material being applied in optical storage, military camouflage, smart windows. Besides that, WO3 can change reversibly from dark blue to transparent which can be used as photochromic switch.





