Tungsten Oxide Thin Film Electrode Cyclic Voltammetry
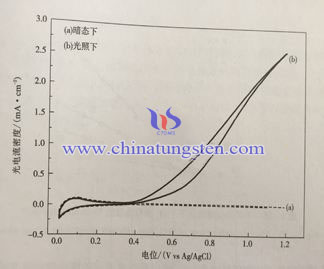
To study the cyclic voltammetry of tungsten oxide thin film electrode, use three-electrode system, sulfuric acid solucion as electrolyte, the property is observed by measuring light current. Below is cyclic voltammograms of WO3 thin film electrode being heat treatment under 450℃ under dark and 500W xenon light source (light strength 100Mw/cm2). We can see that under darkness the polarization current of electrode is small within the scanning range, it is far smaller than anode polarization current under light. Photoelectrochemical reaction under light has good reversibility. Within electric potential 0.35~1.2V(vs.Ag/AgCl), cathode Pt and anode WO3 thin film electrode reaction is as following:
Anode:2OH- + h+ → O2 ↑+ 2H+
Cathode:2H+ + 2e- → H2 ↑
When exposed to light, if the applied bias is low, the Fermi level of WO3 is higher, electrolysis solution accepter is easier to trap photo electron near WO3 electrolyte interface of electrode, so the photo current of anode is weaker, even comes close to 0. With the increasing of bias, Fermi level of WO3 decreases as well, the accepter of electrolyte to trap the photo electron is getting harder which makes the photo electron largely spread to the electric substrate. When bias comes to a certain level, the extra electric filed enlarges the migration rate of photo electron, so photo current of anode strengthens with the shuffling of current.